The story of biotechnology is vast, encompassing numerous fields, but one of the most exciting and transformative areas has been the evolution of gene editing. This niche within biotechnology has revolutionized the way scientists understand, treat, and even prevent diseases. The evolution of gene-editing technologies, from early discoveries to cutting-edge tools like CRISPR-Cas9, showcases both the challenges and immense potential of this field.
The Early Days: Discovering the Power of DNA
The history of gene editing begins long before the concept of "editing" genes became possible. It starts with the groundbreaking discovery of DNA itself in the mid-20th century. In 1953, the work of J Watson and F Crick, along with R Franklin’s X-ray diffraction images, revealed the double-helix structure of DNA. This discovery laid the foundation for future breakthroughs in genetics and molecular biology.
By the 1970s, scientists had begun to unravel the molecular machinery responsible for gene expression and genetic inheritance. These early discoveries paved the way for the development of recombinant DNA technology, where scientists could isolate and manipulate genes. Techniques such as gene cloning and polymerase chain reaction (PCR) allowed for the amplification of specific DNA sequences, but they still lacked the precision needed to truly "edit" the genome.
The First Steps Toward Editing Genes
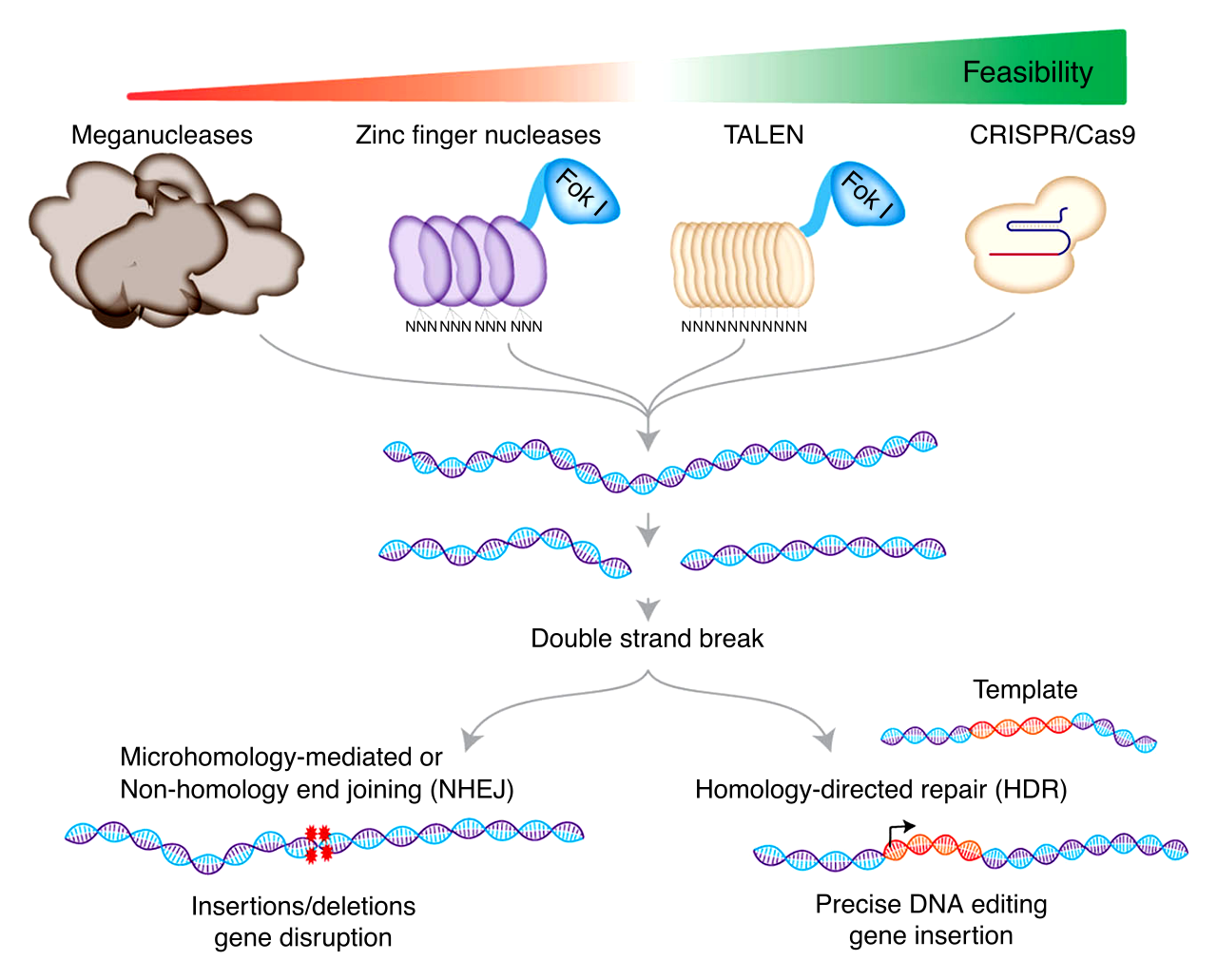
The first true step in gene editing came in the 1990s with the advent of Zinc Finger Nucleases (ZFNs). ZFNs were engineered proteins that could cut DNA at a specific location, allowing for the targeted insertion, deletion, or modification of genes. Though effective, ZFNs were complex and difficult to design, limiting their widespread use in research and clinical applications.
Shortly after ZFNs, another class of gene-editing tools emerged: Transcription Activator-Like Effector Nucleases (TALENs). TALENs were an improvement on ZFNs, providing greater precision in targeting specific genetic sequences. However, TALENs were still relatively labor-intensive and expensive to produce, limiting their use primarily to research laboratories.
While these early tools marked important milestones in gene-editing technology, they still required significant expertise and resources. What the field truly needed was a simpler, more efficient method of gene editing—one that could be widely accessible and transformative for healthcare, agriculture, and beyond.
CRISPR-Cas9: A Game-Changing Breakthrough
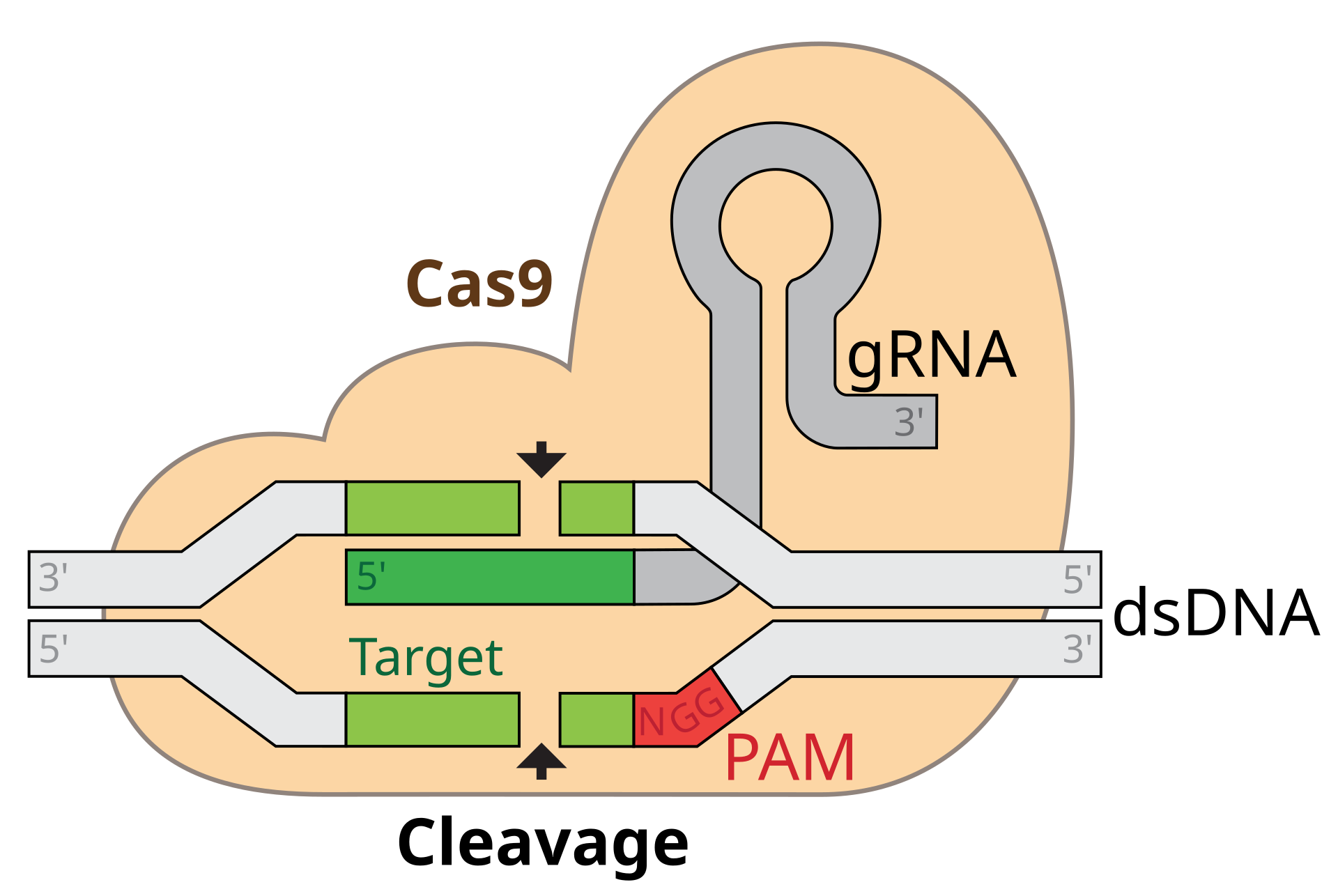
In 2012, a team of researchers led by J Doudna and E Charpentier made an unexpected discovery that would change the landscape of gene editing forever. They found that bacteria use a system called CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) to defend against viral infections. This discovery led to the development of CRISPR-Cas9, a tool that could be used to edit the DNA of almost any organism with remarkable precision.
CRISPR-Cas9 works like molecular scissors, cutting DNA at a specific location in the genome, allowing scientists to add, remove, or modify genes with unprecedented ease. Unlike ZFNs and TALENs, CRISPR-Cas9 is easy to design, inexpensive, and highly efficient, making it accessible to researchers and clinicians around the world. The simplicity of CRISPR-Cas9 has democratized gene editing, allowing scientists to perform experiments that were once considered impossible.
Revolutionizing Medicine: From Gene Editing to illness Treatment
The success of CRISPR-Cas9 in research quickly extended to practical applications in medicine. In 2015, scientists used CRISPR to correct a genetic mutation responsible for a rare disease called Duchenne muscular dystrophy in mice. This achievement opened the door to the potential for treating genetic disorders in humans.
By 2017, researchers had made significant progress in using CRISPR to treat sickle cell anemia, a genetic blood disorder that affects millions worldwide. In early clinical trials, patients with sickle cell disease underwent gene editing to correct the mutations in their hemoglobin-producing genes. The results were promising, with some patients showing remarkable improvements in their symptoms.
CRISPR also holds immense potential in the fight against cancer. By editing immune cells, researchers have been able to enhance the body’s ability to recognize and attack cancer cells. Early trials using CRISPR-edited immune cells, such as T-cells, have shown encouraging results in patients with leukemia and lymphoma, opening up new possibilities for immunotherapy.
Moreover, CRISPR’s potential extends to preventing diseases before they even arise. In 2020, a research team in China made headlines when they used CRISPR to edit the embryos of human babies to make them resistant to HIV. This controversial experiment sparked ethical debates but also highlighted the future possibilities of gene editing to prevent inherited diseases before birth.
Biotechnology in Agriculture: Improving Crop Resilience and Yield
Gene editing has also had a profound impact on agriculture. With the global population expected to reach nearly 10 billion by 2050, there is an urgent need to increase food production while also ensuring sustainability. CRISPR-Cas9 has enabled scientists to develop genetically modified crops that are more resistant to pests, diseases, and environmental stress, such as drought.
In 2016, a team of scientists used CRISPR to create a strain of rice that was resistant to a bacterial disease called bacterial blight, which costs billions of dollars in crop losses annually. The following year, researchers edited the genome of wheat to make it resistant to wheat rust, a disease that devastates wheat crops worldwide. These advances promise to increase crop yields, reduce the need for harmful pesticides, and contribute to food security.
Furthermore, CRISPR has the potential to create crops with enhanced nutritional profiles. In 2020, researchers developed a strain of wheat with higher levels of a key nutrient, folate, which could help address global deficiencies in this essential vitamin.
Ethical and Regulatory Challenges
Despite its immense potential, the rise of gene-editing technologies has not been without controversy. The ability to edit human genes, especially in embryos, raises ethical concerns about "designer babies" and the possibility of unintended consequences. In response, regulatory bodies around the world have called for stringent guidelines and oversight to ensure the responsible use of CRISPR and other gene-editing tools.
In the United States, the Food and Drug Administration (FDA) has been cautious in approving gene-editing therapies for clinical use, while other countries, like China, have moved ahead with trials involving gene-edited embryos and genetic modifications for disease prevention. These differences in regulatory approaches have led to debates about how to balance scientific progress with ethical considerations.
Moreover, while CRISPR-Cas9 has proven to be a highly effective gene-editing tool, it is not without its limitations. Off-target effects, where the tool makes unintended changes to the genome, remain a concern. Researchers are actively working on improving the precision of CRISPR and developing newer, more refined versions of the tool, such as CRISPR-Cas12 and CRISPR-Cas13, to address these issues.

Looking to the Future: The Next Frontier of Gene Editing
As we look to the future, the possibilities for gene editing seem limitless. Beyond human medicine and agriculture, gene-editing technologies could transform environmental conservation. For example, scientists are exploring ways to edit the genomes of endangered species to make them more resilient to climate change. Similarly, gene editing could help address environmental issues such as the restoration of coral reefs and the reduction of carbon emissions through the modification of plants and microorganisms.
In the coming years, we are likely to see more clinical trials using CRISPR to treat a wide range of genetic disorders, from cystic fibrosis to Huntington's disease. The ability to correct genetic mutations at the DNA level could offer hope to millions of people suffering from inherited diseases.
Moreover, with ongoing advancements in synthetic biology and gene editing, we may even see the development of entirely new organisms designed to perform specific tasks, such as producing biofuels or breaking down pollutants in the environment.
A New Era in Biotechnology
The story of gene editing is a testament to the power of science and innovation. What began as a distant dream in the realm of molecular biology has now become a cornerstone of modern biotechnology, with the potential to reshape medicine, agriculture, and the environment. The evolution of gene-editing technologies—from the discovery of DNA to the revolutionary CRISPR-Cas9 tool—marks a new era in biotechnology, one where the boundaries of what is possible are continually being pushed.
While challenges remain, particularly in terms of ethical considerations and regulatory oversight, the future of gene editing holds immense promise. As scientists continue to refine these technologies and explore new applications, gene editing will undoubtedly play a pivotal role in solving some of humanity’s most pressing problems, from curing genetic diseases to feeding a growing global population. The success story of gene editing is still being written, and its impact on the world will continue to unfold for generations to come.
Key Resources